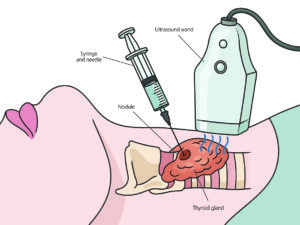
The Bethesda System for Reporting Thyroid Cytopathology is a standardized classification system that is used to interpret and report the results of thyroid fine needle aspiration (FNA) biopsies. The system was developed to improve the accuracy and consistency of reporting, and to guide the management of patients with thyroid nodules. Only cells from a biopsy can be used to make an assessment.
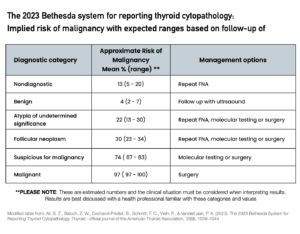
The Bethesda System categorises thyroid FNA biopsy results into six diagnostic categories based on the type and degree of abnormality detected in the cells:
Bethesda category 1 – Non-Diagnostic
The biopsy sample is inadequate for diagnosis because of a lack of cells in the biopsy. This may mean another biopsy will need to be performed.
If the nodule is a pure cyst (a nodule containing only fluid), the biopsy will fall into this category as there are no cells to make a diagnosis.
Bethesda category 2 – Benign
The cells are normal and show no evidence of cancer or abnormal growth. This is the most common category and accounts for approximately 85% of all biopsies.
Bethesda category 3 – Atypia of Undetermined Significance
The cells show some abnormalities, but the significance of these changes is uncertain. Nodules in this category have approximately a 20% risk of being cancerous.
Bethesda category 4 – Follicular Neoplasm
The term “neoplasm” is sometimes misinterpreted to mean a cancer. The word “neoplasm” originates from Ancient Greek and literally means “new growth”. This may mean a benign, non-cancerous growth or a malignant, cancerous growth.
Biopsies in this category tell us that the nodule may be benign or cancerous, but we cannot confirm this from a biopsy alone. Surgery to remove the part of the thyroid with the nodule is usually recommended, as there is a risk of cancer of up to 30% with this type of nodule. Molecular analysis of nodules in this category can be done to arrive at a more definitive diagnosis (without surgery), but it is not available in Australia. However, biopsy samples may be sent to the USA for analysis at the patient’s cost.
Bethesda category 5 – Suspicious for Malignancy
The cells show features suggestive of thyroid cancer, but the diagnosis cannot be confirmed definitively.
Bethesda category 6 – Malignant
The cells show clear evidence of cancer.
The Bethesda System helps to standardize the reporting of thyroid FNA biopsy results and provides guidance on the management of patients with thyroid nodules based on the diagnostic category. The system has been widely adopted and is considered an invaluable tool in the diagnosis and management of thyroid nodules.
Biopsy results should always be considered as part of the overall assessment of the nodule and not a stand-alone result. There is a recognised false negative rate associated with all biopsies – where a biopsy result is negative for cancer, but it is false/wrong. For thyroid biopsies, this rate is approximately 5 – 10% and up to 30% in very large nodules (>4cm).